
"Synthesizing Ideas On Formation of ionic Bond" Wikipedia July 14 2008.
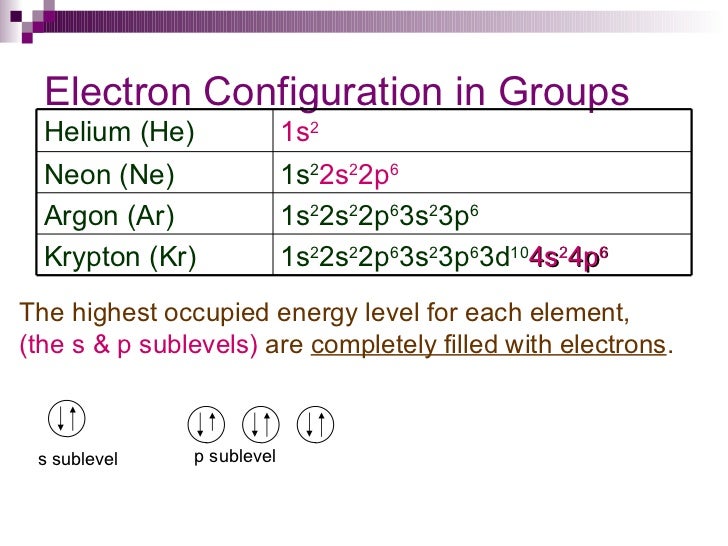
Since "5s 2" was not included in Krypton's electron configuration, we need to write that down.ġ. So, Strontium's simplified electron configuration is the following: Since Krypton's electron configuration is included in Strontium's electron configuration, we can replace "1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p6" with Krypton's symbol, which is Kr. That is, both Strontium and Krypton have the following in their electron configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6. Kr 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6Īs you can probably see, most of the Strontium's electron configuration is covered by Krypton's electron configuration. The electron configuration of Krypton, Kr, is: Strontium's closest Noble Gas is Krypton, Kr. The electron configuration of Strontium, Sr, is:
#Krypton electron configuration how to
Now that we know what Noble Gases are, we are finally able to learn about how to simplify the Electron Configuration of an element. How do we use Noble Gases to simplify the Electron Configuration of any Element? Here's what the atom of Fluorine originally looked like ( the black dots represent the electrons in the outermost shell): Fluorine needs to gain an electron to have the same core configuration of that of Neon. For example, the atomic number of the element Fluorine, F, is 9, which means that its closest Noble Gas is Neon, Ne. To figure out which Noble Gas an element is closest to, look at its atomic number. Therefore, all elements, other than the Noble Gases, can gain or lose electrons to have the same core configuration of that of the NEAREST Noble Gas. For example, if an element X has 6 electrons on their outermost shell, and the outermost shell needs 8 electrons to be considered full, element X needs to GAIN 2 electrons to make its outermost shell full. To make these electrons stable, they either need to LOSE or GAIN electron(s) on their outermost shell.

#Krypton electron configuration full
As you can see, all of Neon's shells are full because the first shell of any element can only hold 2 electrons, and the second shell of any element can only hold 8 electrons.Īll other elements in the Periodic Table are not stable because not all of their shells are full of electrons.


 0 kommentar(er)
0 kommentar(er)
